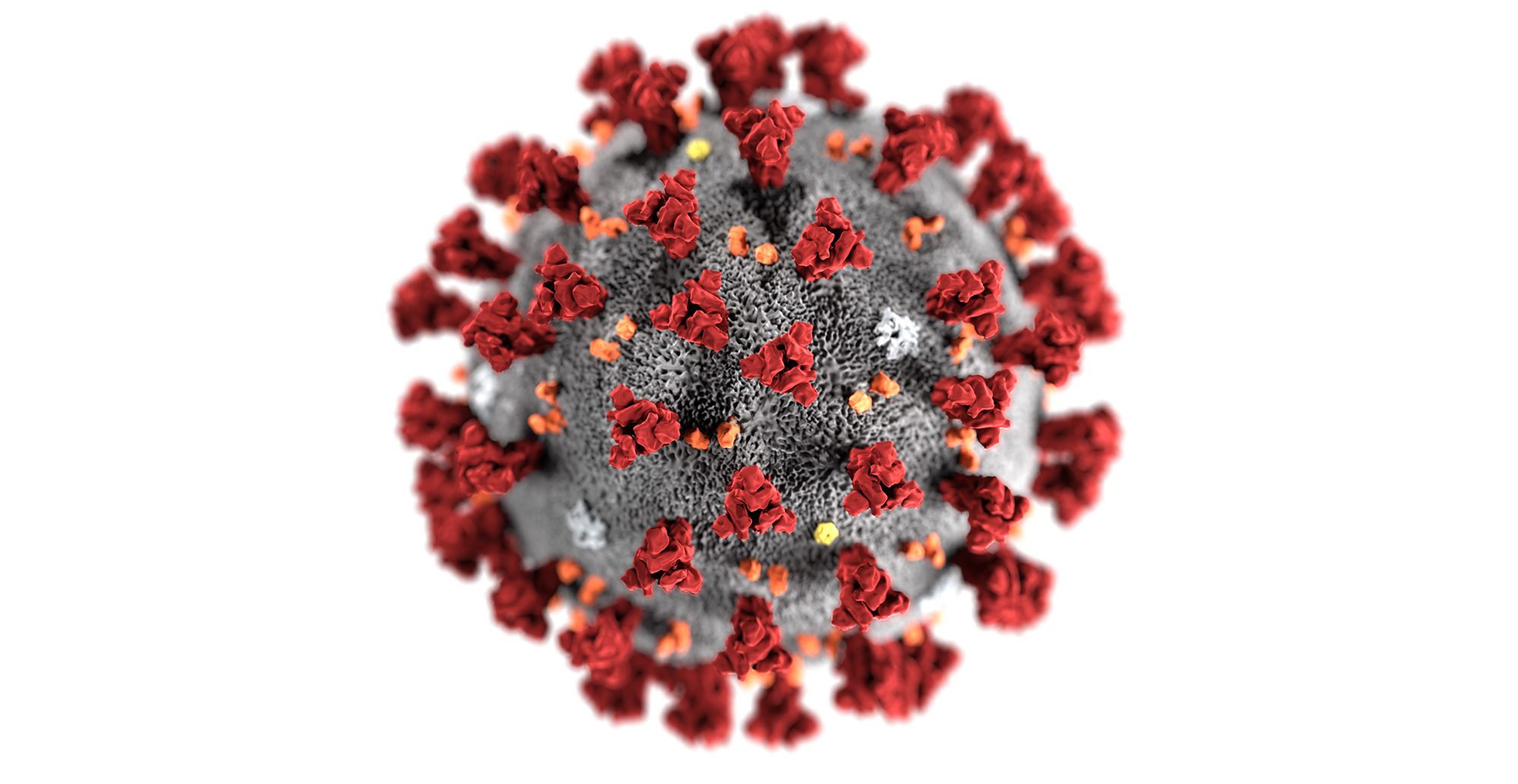
COLA is engaged with the CDC’s Laboratory Outreach Communication System (LOCS) in the Division of Laboratory Systems (DLS) to transmit their public health messages to our COLA laboratories and affiliates. Below is a link to the most recent LOCS message about the FDA suspension of review of EUA requests for Laboratory-Developed Tests for SARS-CoV-2.
FDA Suspends Review of EUA Requests for Laboratory-Developed Tests for SARS-CoV-2
The U.S. Department of Health and Human Services (HHS) recently announced that the U.S. Food and Drug Administration (FDA) will not require premarket review of Laboratory-Developed Tests (LDTs) for SARS-CoV-2. In alignment with this announcement, FDA will not review Emergency Use Authorization (EUA) requests for any LDTs at this time.


.png?width=261&name=2021_newest_logo_cola-footer%20(1).png)